Improving Lives & Restoring Health with Innovative Allograft Solutions

Understanding Our Purpose
Pinnacle Transplant Technologies is a multi-service allograft tissue bank

OUR PRODUCTS
Allograft Portfolio
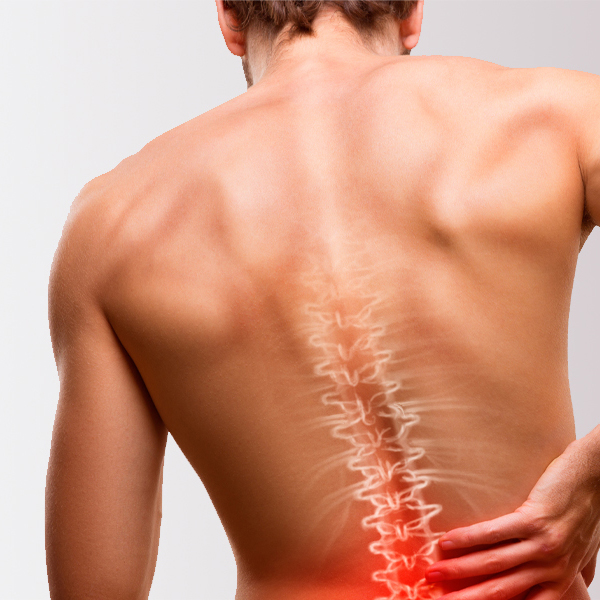
Spine Allografts
Precision-machined grafts, demineralized bone matrix/fibers (DBM/DBF) and allograft chips
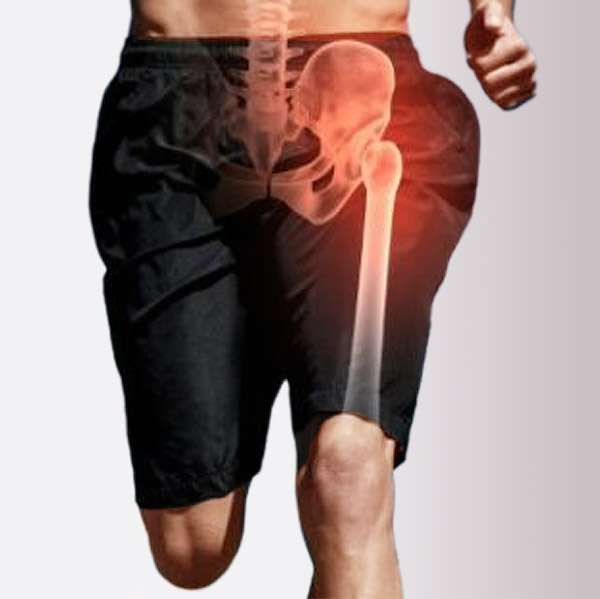
Sports Medicine Allografts
Soft tissue allografts, with and without bone
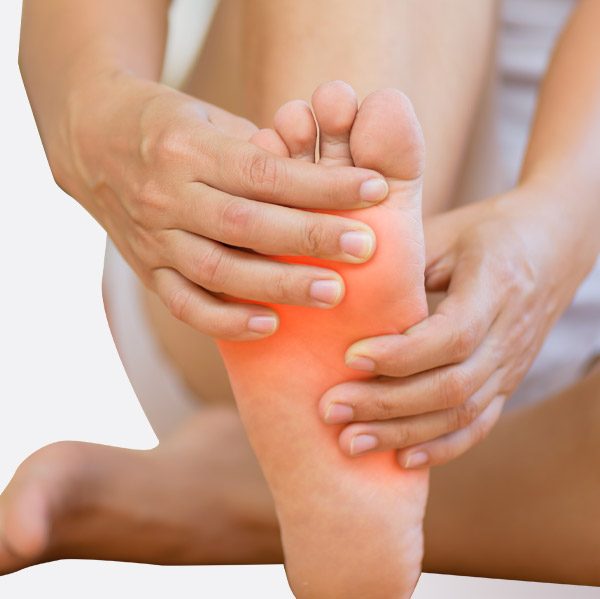
Wound Care Allografts
Biological overlay for wound healing
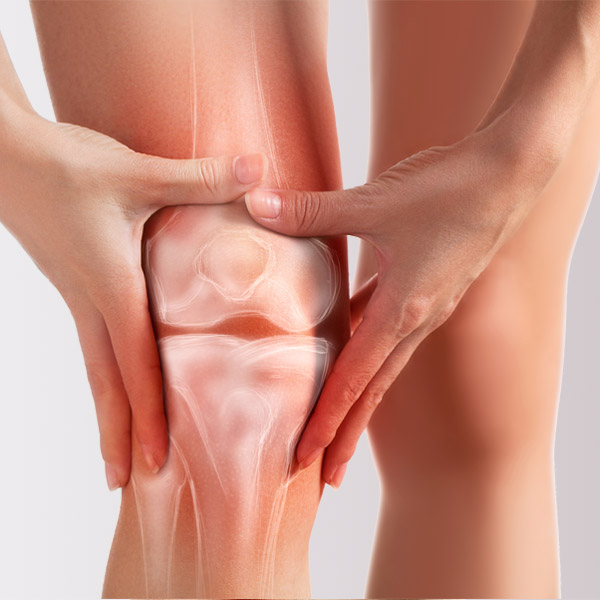
Orthopedics Allografts
Versatile solutions for a wide array of orthopedic applications
To ensure allograft safety & quality, Pinnacle prepares donated tissue for transplantation through extensive testing and screening, and validated sterilization processes. Learn More…

Your Allograft OEM Partner
Private-Labeled and Custom Allograft Opportunities
Pinnacle provides contract manufacturing opportunities, and private-labeled and proprietary implants, such as, DBM putties, fiber, sponges, particulate/chips, soft tissue, structural grafts and amniotic membranes. We are dedicated to working with customers to develop innovative, value-added products.
The Greatest Gift
Whats New
News & Stories
No posts were found for provided query parameters.
Contact Us
Pinnacle is committed to honoring the gift of donation and improving patient’s quality of life through the processing and distribution of high-quality allograft implants.
Customer Service
1125 W. Pinnacle Peak Road, Building #2
Phoenix, AZ 85027
Phone: 623-277-5400
Fax: 623-277-5401
info@pinnacletransplant.com
To request more information or contact a representative from Pinnacle’s Team, please complete the form below

© 2023 Pinnacle Transplant Technologies, All Rights Reserved
Navigation
Location & Contact
- 1125 W. Pinnacle Peak Road, Building #2
- Phoenix, AZ 85027
- Phone: 623-277-5400
- Fax: 623-277-5401
- info@pinnacletransplant.com
